Asacolon®
Information placed on this website is not intended as a substitute for consultation with your healthcare professional. Please consult your healthcare professional for further information.
Asacolon® Tablets are prescribedꝉ as a first-line treatment for mild to moderate acute ulcerative colitis (UC) and for the maintenance of remission
(Asacolon® Tablets (400mg and 800mg) for use in adults and children over six years; Asacolon® Tablets (1600mg) for use in adults only1-3)
Asacolon® Suppositories are prescribed for acute mild to moderate ulcerative colitis limited to the rectum (ulcerative proctitis) in adults4
Important information on Asacolon® and its use can be found in the Summary of Product Characteristics – the SPC and PIL are meant for HCPs or patients in Ireland who have already been prescribed the product
You can also find the Patient Information Leaflets for Asacolon® here
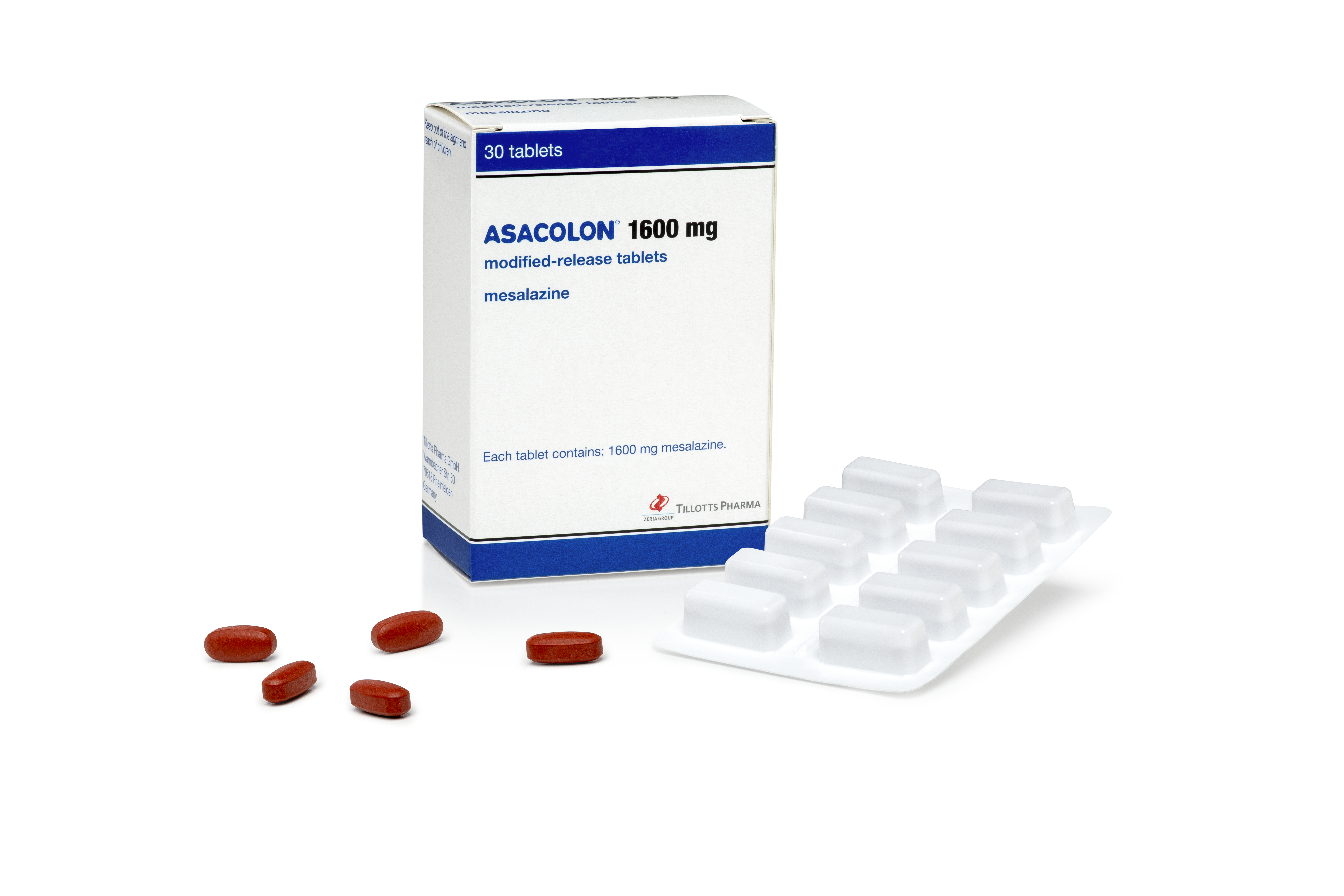
Asacolon® 1600mg
Asacolon® 1600 mg (mesalazine) incorporates the new OPTICORE™ technology and is indicated for the treatment of mild to moderate acute disease and for the maintenance of remission.
Important information on Asacolon® 1600 mg and its use can be found in the Summary of Product Characteristics (SPC and PIL)
Last update: November 2023
All trademarks used or mentioned here are protected by law. Tillotts Pharma AG’s trademarks include Tillotts®, Asacol™, OPTICORE™, Octasa™, Fivasa™, Yaldigo™, Kiudro™, Lixacol™, Asacolon™, Colpermin™, VistaPrep™, Entocort™, Entocir™, Entocord™, Budecol™ and DIFICLIR™ are either registered or applied for in around 65 countries (transfer of registrations to Tillotts pending in certain countries). The rights to Asacol, including the rights to the trademark, are owned by Tillotts in various countries except for the following: Switzerland, USA, United Kingdom, Canada, Italy, Belgium, the Netherlands and Luxembourg. The product rights to Asacol 1600 mg are owned by Tillotts globally, with the trademark varying from country to country. OPTICORE™ has been developed based on the Phloral™ technology.
Product information on this website is limited, with the aim of providing a summary for general information purposes for HCPs and patients regarding the activities of Tillotts, and could contain product details or information not accessible or valid in your country. Tillotts disclaims responsibility for access of information which may not comply with any regulation or standard in any particular country. More information may be available from local regulatory authorities, but not all products are available in each country. Please consult a healthcare professional for further information.
References
1 Asacolon 800 mg Gastro-Resistant Tablets Summary of Product Characteristics
2 Asacolon 400 mg Gastro-Resistant Tablets Summary of Product Characteristics
3 Asacolon 1600 mg modified-release tablets Summary of Product Characteristics
4 Asacolon suppositories 500mg & 1000mg Summary of Product Characteristics


